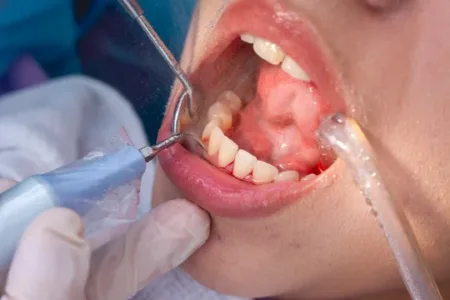
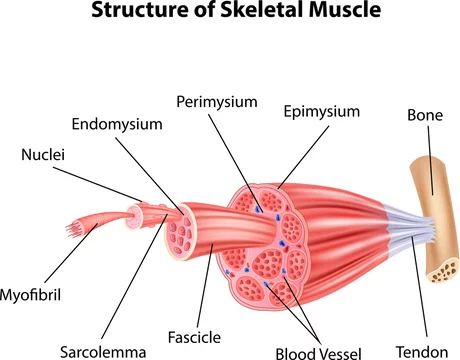
Cheer P 18 Precision is a significant aspect of today’s dental practice to offer quality services to clients. From accessing cavities to adjusting crowns, specially designed instruments are used…
If you’ve ever hit the gym after a bit of a break or pushed yourself a little harder during a workout, chances are you’ve felt the tell-tale ache the next…
California is home to some of the most spectacular stargazing locations in the country, with its vast desert landscapes, high elevations, and protected dark sky areas. Whether you’re in the…
In recent years, resistance bands have surged in popularity, becoming a staple for athletes, fitness enthusiasts, and even physical therapists. Their versatility and convenience make them a favorite for both…
GE window air conditioners, like the AEE08AT model, offer excellent options for effectively cooling spacious areas, even large rooms and extra-large spaces, on sweltering summer days. Obstructed filters, power-related concerns,…
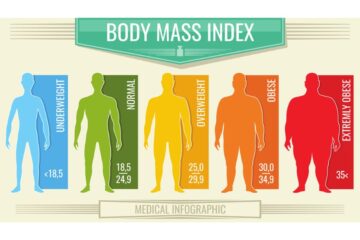
When discussing weight loss and health, understanding your Body Mass Index (BMI) is crucial. The simple act to calculate your BMI can be a transformative first step. And while there are several methods to tackle weight-related issues, one unique solution stands out:…
Key Highlights Discover the importance of early training and its impact on your puppy's development. Learn effective positive reinforcement techniques and why they're preferred over punishment-based methods. Familiarize yourself with essential equipment like…
Pursuing higher education can be a significant financial burden for many Nigerian students. To alleviate this challenge, the Nigerian government has introduced the Nigerian FG Student Loan Scheme. This initiative aims to provide financial assistance to deserving students, ensuring that financial constraints…
Aging is a natural process, but that doesn’t mean your skin has to show its effects prematurely. Collagen is the key to maintaining youthful, firm, and plump skin. As we age, collagen production…
Weight loss programmes totally exclude carbs and lipids from each meal. Limiting certain nutrients is important, but eliminating them entirely might impair your body's natural processes and metabolism. People attempt various diets every day in an effort to maintain their optimum body…
In an era dominated by digital platforms, the internet has become a double-edged sword, offering vast opportunities for learning, connection, and entertainment, but also presenting significant challenges to mental health. The digital age,…
Let's talk about a universal style puzzle we've all faced: finding that one wardrobe piece that's effortlessly cool, suits every occasion, and ages like fine wine. Well, here's the secret…
The comfort level of cotton panties for ladies can vary depending on personal preferences and individual body types. It offers a wide range of products, including clothing, electronics, accessories, and…
Nella has always judged men based on their appearance. And when I mean appearance, I mean from the shirts to their trousers and even their boxer briefs. Crazy right? She…